Montgolfier's pumping fire engine
An astonishing machine that paved the way to the furnace air engine
History
The Montgolfier brothers are known as the inventors of the Montgolfière hot air balloon. They did many other inventions as for example the self-acting hydraulic ram in 1796. In 1784 they patented - or better said - they officially deposited a letter (called un paquet) at the Académie Royal containing their invention about a pumping fire engine.
Very curious by nature, the Montgolfier brother reflected on the causes of the prodigious effort with which the gunpowder drives out the ball as it ignites, as well as the promptness with which the barrel heats up. After several experiments they were convinced that the flammable gases and the air contained in the powder were the the two factors that constitute the force at the time of the deflagration. All this seems very logic nowadays, but at their time the law of thermodynamic did not exist at all.
From their experiences, they concluded that it is possible to raise water on hillsides with simple and economical means.
Engine arrangement
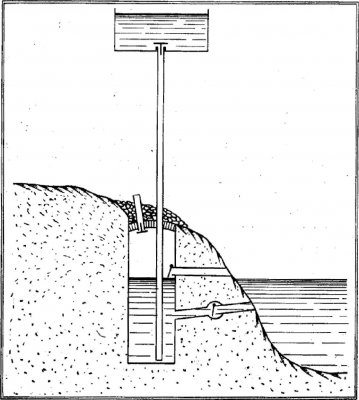
A well of large diameter has its bottom a few feet below the level of the river or pond whose water is to be raised. The well contains water and air. The well is arched from above. This vault is above the water level at a height at least as large as the diameter of the well. A charge is placed on the vault whose weight is according to the height at which it is proposed to raise the waters.
The well communicates with the external environnement by the means of 4 pipes.
An upper reservoir will receive the waters from the river and a large diameter vertical pipe allows communication between this reservoir and the well. It is positionned at the bottom of the tank and goes inside of the well, below the level of the water, until the bottom. A non-return valve positionned at the top of the pipe prevents water raised to the reservoir from going back into the well.
Another communication pipe connects the river to the bottom of the well. This pipe allows the water of the river to enter the well. On it is placed another non-return valve which prevents water introduced into the well, to go back to the river once the air pressure within the well in increased.
Finally two other pipes are positioned to change the air.
The first is a vertical air pipe open at both ends. It communicates from the inside top of the vault to the outside by crossing the thickness of the vault and its load. This pipe is closed at the internal side by a plug that can act as a valve preventing the air inside of the the well to go out when the pressure is increased.
The other one is horizontal air pipe also open at both ends. It is at a level of about half a foot above the water level and communicates from outside to the inside of the well. A lightweight valve prevents the air from leaving the well at the time of the pressure increase.
This horizontal pipe is large enough to allow the machine operator to introduce combustible into the well.
Operation
Small bundles of not tight, driest and most rapid burning wood are placed by the machine operator into the well, at suitable distances, on a grate (not shown in the picture) positionned between the ends of the two air pipes (vertical and horizontal). The bundles are then ignited all at the same time by means of fire or any other mean.
The wood combustion heats up the air whose pressure increases dramatically. Once the air pressure exceeds the pressure that corresponds to the height of the water column within the vertical pipe, it starts to expand and the water flows toward the upper reservoir. As a result of the air expansion, the air pressure decreases and the raise of the water occurs until the pressure of the air and the water colum equalize.
After the air expansion the water level within the well is below the water level of the river.
After pressure equalization, or better said, once the water has stopped to raise, the operator pushes the plug of the vertical air pipe and allow the hot air to escape to the atmosphere. The water of the river being at higher level rushes into the well, pushing out the hot air. Once water levels are equalized and the water stops entering the well, the hot air keeps on going replaced by cold air entering from the horizontal air pipe. As the density of cold air is higher than the one of hot air, the latter one is completely evacuated from the well.
Once this is done, the operator starts the operation again.
According to the tests that have been made, it can be thoroughly state that - with such a machine - it is possible to raise 1,200 thousand cubic feet of water per day to the height of 50 feet for a consumption of about 100 quintals of wood.
There is room though to further reduce the wood consumption.
One foot = 0.325 m.
One cubic foot = 0.0344 m3.
One quintal = 100 pounds = 48 kg.
NB : Units at that time differed from one country to the other.
The figures formulated by Montgolfier give an idea of the power envisaged and the expected returns.
If we translate them into metric units we find that the machine will develop an effective power of 100 hp, at the cost of an hourly consumption of 200 kg of wood.
The potential energy gained per day by the raised water is equal to : Water density x gravity x height x number of cubic meter per day
Translation into number gives: Energy = 1000 x 9.81 x (50 x 0.325) x (1 200 000 x 0.0344) = 6 580 548 000 J per day or 6 580 MJ per day
The machine power is obtained by dividing the result by the number of second per day (1J = 1W/s):
Machine power = 6 580 548 000 J / 86 400 s = 76 163.75 W or about 101 HP
The wood comsumption is 100 quintals per day, which is 100 * 48 kg = 4800 kg of wood per day or 200 kg per hour
As a consequence the machine gives 100 horse-hour for a consumption of 200 kg. This is a consumption of 2 kg of wood for 1 horse-hour produced.
The combustion of 1kg of wood will give about 15 MJ of energy (depending on the quality of the wood).
The machine consume 4 800 kg of wood per day, that is 4 800 kg x 15 MJ = 72 000 MJ of energy per day.
With this daily energy consumption it produced 6 580 MJ of energy per day.
Therefore its performance is 6580 MJ / 72 000 MJ = 9.14%.
This is at the level of the best steam engines ever made!
These results are properly magnificent for the time.
Experiments
Such remarkable results have been followed by Montgolfier's other spectacular experiments with estonishing results. More importantly they bring the proof of their effective realization; a very important point. The results written in his letter of the 17th of May 1784 speaks for temselves:
The combustion of the sixth part of a grain of wax absorbed about the sixth part of the air contained in a glass vessel with a capacity of about 14 cubic inches but it did not provide any significant pulsation.
The burning of 12 grains of paper in a terracotta vessel with a capacity of about 200 cubic inches produced a pulse of 1 pound weighing water at more than two feet in height.
That of 1 large piece of paper in a tin vessel with a capacity of about 1200 cubic inches produced a pulsation of 12 pounds of water at more than 6 feet in height.
Finally that of 2 ounces of paper in a tin vase with the capacity of about 4 cubic feet produced a pulsation of about 120 pounds of water at a height of almost 8 feet but this last experiment did not produces its full effect because of the losses of the vase.
NB: 1 grain: 0.053 gram; 1 gros = 72 grains = 3.82 grams; 1 ounce = 8 gros = 30.59 grams; 1 pound = 16 ounces = 489.5 grams; 1 inch = 27.07 mm; 1 foot = 0.325 m.
The second experience of this list gives following results.
The energy gain in raising water is : Water density x gravity x height x volume of water
1 kg of water = 1 liter
In numbers this gives: 1000 x 9.81 x (2 x 0.325) x (1 x 489.5 / 1000) = 3121.29 J
The energy given by the paper combustion is somewhat equal to the one given by the wood : 15 MJ per kg
The energy given by the 12 grain of paper is : 12 x 0.053 / 1000 * 15 MJ = 9 540 J
The performance was : 3212,3 J / 9540 J = 33.67 %
This is as performance as an internal combustion engine nowadays.
The last experiment:
The energy gain in raising water is : 1000 x 9.81 x (8 x 0.325) x (120 x 489.5 / 1000) = 1 498 222 J
The energy given by the combustion of the paper is : 2 x 30.59 / 1000 * 15 MJ = 917 700 J
Performance is : 61.3% !
Montgolfier says : this last experiment did not produces its full effect because of the losses of the vase...
With regards to the Carnot law, these results seems a bit overstated if the combustible that was used has been ordinary paper that burns at 250-300°C. The paper used in these experiments must have been of such quality that burns at high temperature. It is absolutely possible as Montgolfier and his brother were running a paper manufacture, and this can be the explanation for using paper as a combustible.
Finally, experiments are not real engines. This said, Montgolfier invention was a true novelty for its time and the man was already fascinated by the recent discovery of flammable air (hydrogen recently discovered by Cavendish) and felt that it will serve wonderfully the purpose of the fire pump.
Sources
| Sources | Author | Date | Title |
|---|---|---|---|
| Histoire de l'académie royale des sciences | 1804 | Sur la machine de M. de Montgolfier | |
| La Nature | Charles Cabanes | 1936 | Joseph de Montgolfier - Inventeur du moteur à combustion interne |