Shaw's Hot Air Engine
Source: an article from Scientific American, Vol IX, N° 42
Date: July 1st, 1854
Title: Shaw's Hot Air Engine
The 1854 Shaw engine, is a hot air engine of a new type.
Unlike is predecessors, Philander Shaw took into account the physical fact that the air is a bad conductor; which means that it heats up slowly. In usual air engines like the Stirling engine, the air that comes from the cold side to the hot side, has little time to heat up. If the engine rotates at 120 rpm, the batch of cold air will have only a quarter of a second to heat up (included the running through the regenerator).
Shaw built a special heater where the air was stored and heat up with time before entering the acting piston. This was indeed a superior improvement and allowed the Shaw engines to be more powerful.
A later shaw engine, exhibited at the Paris exhibition in 1867, was a 20 hp air engine.
To economize on fuel, Shaw did not use a regenerator but effective common sense. He used the expanded air to feed the fire, and doing so effected considerable saving of fuel.
Confusion should be avoided. The Shaw engine was not a furnace air engine, but an external combustion air engine. Although the working fluid was mixed with the product of combustion, it did so after having acted upon the working piston with the sole purpose to take less heat to the combustible and not to gain in pressure and then expand.
This engine had nevertheless some drawbacks.
It was a intricate engine, with many parts, though increasing its fragility and side effects like friction and performances losses.
Moreover there was a non explainable feature: the working fluid in the compression chamber was cooled down.
In an air engine the cooling happens during the compression, in order to save work given to the fluid. But once the compression is done, there is no reason to cool the working fluid.
Why Shaw did such a feature remains mysterious.
Finally, Shaw brought up a good machine, complicated, but working, at a time when the Ericsson adventure finished by a tremendous failure and Stirling had abandonned the building of hot air engine.
The annexed engravings are views of improvements in Hot-Air Engines, invented by Philander Shaw, of East Abington, Mass., a patent having been granted to him on the 2nd of last month (May, 1854).
These engravings, however, represent a modification and arrangement of some parts different from that described in his patent, and believed to be improvements, while he has retained all the principal features claimed in the patent.
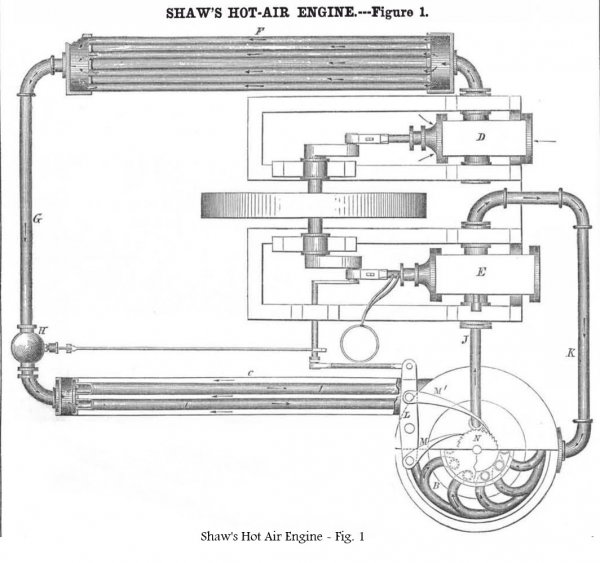
Figure 1 is a top view of the whole apparatus (the cylinder being an oscillating horizontal one) showing the air compressing chamber, the entrance heating tubes, and the final heating tubes in section. Fig. 2 is an elevation, partly in section, of the air heater. The same letters refer to like parts on both figs.
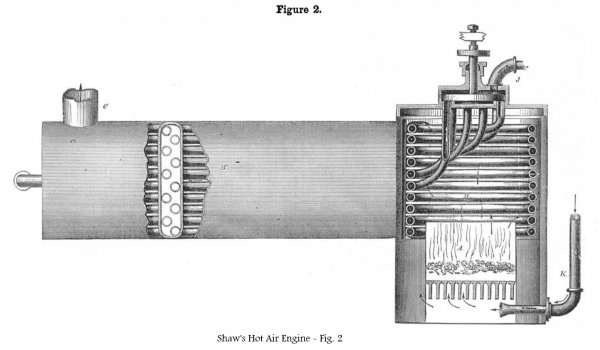
A is the furnace; the heated products of combustion pass up on the outside of the final air-heating tubes, B, through the tubes in B’, and then through the smoke pipe, C, in which are the entrance air-heating tubes, I.
D is the feed air pump, and E is the main cylinder, in which is the working piston operated by the hot-air. The air pump, D, takes in air from the atmosphere, and forces it into the compresser, F, where it is maintained at 60 lbs. on the square inch (approx. 4.1 bars).
From the compresser, F, it is admitted into the tubes, I, in the smoke-pipe through the pipe, G. There is a valve in the pipe at H, which cuts off and lets in the air to the tubes, I.
The heater, B, is composed to a series of tubes, forming a coil, which are connected with a perforated rotating top-plate moved round by the vibrating beam, L, which operates the ratchets, M, M’, which take into the teeth of the ratchet wheel, N, secured on the cap of the rotating heater coil, B.
The air fed into the tubes in the smoke-pipe, takes up some heat from the escaping gases, and is admitted by rotation into the several pipes of the main heater furthest from the fire, while each tube in the coil which receives the concentrated heat of the fire, contains the exact quantity of air to be admitted into the main cylinder each stroke; then for the next stroke the top plate is moved one notch, and brought to communicate with another tube and to the steam box and cylinder, E, and so on continually.
The object of the inventor by this heater is to give time to the air to become heated, and not take in a fresh quantity of cold air to be heated at once under the piston of the main cylinder. This method of heating the air apart and separate from the main cylinder is certainly a superior plan, and the means for giving the air a long heating circuit from the time it enters the smoke-pipe tubes to its final admission into E, is very ingenious.
It will be observed that the hot air, after acting upon the piston, is employed to feed the fire. It is exhausted through the pipe, K, and passes up through the grate, as shown in fig. 2. This is a good idea and must effect a considerable saving of fuel.
The piston is kept cool, and the packing preserved from being burned out by a stream of water admitted through the hollow piston rod by tubes, as shown, and which circulates through the piston which is also hollow.
The higher the air becomes elevated in temperature its pressure increases, therefore as it receives its concentrated heat of the fire in the coil heater, B, its pressure is far higher there than where it is injected into the entrance heating tubes, I.
The advantage of this arrangement is, that it relieves the engine from working against the highest back pressure in feeding in the cold air, as it is fed into the feeding apparatus, where the temperature is comparatively low, while it is taken into the main cylinder, E, at its very highest temperature and pressure.
The heads of the coiled pipes of the heater, B, are inserted close to the top plate, this latter acting the part of a rotating disk valve. It is intended to have a stream of cold water circulating through the compresser, F, so as to carry off the heat of the air developed by compression, and thus have the air in as condensed a state as possible when it enters the heater.
We cannot see the advantage to be derived from thus reducing the temperature of the air when that same temperature has to be given to it again — first cooling and then heating the air before it is used.
The main cylinder is 2006 inches area (approx. 1.29 m2), and that of the pump 1209, area (approx. 0.78 m2); the stroke of both is two feet (approx. 61 cm).
The power of this engine will be according to the quantity of air heated in a given time, and the temperature to which it is raised, — in other words, the pressure and velocity. The heat applied imparts the quality of expansion to the air. Expansion is the force of hot air and it is measurable in quantity, the same as the force of gravity, — the quantity of water which falls in a given time through or down a certain length of space.
Thus 491 volumes of air will expand to 982 — double the volume — when it becomes heated to 491° F (approx. 255° C), and at this temperature will exert a pressure of 15 lbs. on the square inch (1 bar). This degree of heat is too high to be used in an engine, it would be impossible to keep the piston lubricated while exposed to such a temperature. The main cylinder, E, contains 27.85 cubic feet (approx. 790 l) of air, and the feed pump, D, has a capacity of 16.79 cubic feet (approx. 475 l).
To make the calculation easier, but not the less plain, let us assume that the capacity of E is 28 cubic feet (approx. 793 l), and that of D 16 (approx. 453 l) — the difference being 12 (approx. 340 l) or three-sevenths in favor of E, against the feed pump, D.
As the large cylinder can only receive one pump full from D every stroke, however much it may condense the air in F, it follows that the average pressure in E, during the stroke, if the air is heated to 491° F (approx. 255° C), will be 15 - (6 3/7) = 8 4/7 lbs. on the square inch (approx. 0.59 bar) during the stroke. If the air could be heated to give 50 strokes per minute, the power of the engine, would be 2006 x (8 4/7) x 100 / 33,000 = 52.10 horse power.
But then to do this the heater must be able to heat 600 cubic feet (approx. 17 m3) of air to 491° F (approx. 255° C) above its atmospheric temperature every minute. The “Ericsson” engine made only 19 strokes (semi-revolution of crank) per minute, when we saw them in operation.
The great bulk of air to be operated upon in an air engine, is the great obstacle to its use.
The fact is here revealed to us plainly, that it is impossible to use condensed air in air engines, when the feed pump is only equal or less than the main cylinder. It requires the feed pump to be of greater capacity than the main cylinder to do this.
The new “Ericsson” engine, in which highly compressed air, was stated to be used, were delusions, because the feed pumps were of less capacity than the main cylinders. The quantity of hot air admitted into the main cylinder every stroke, and its temperature, are the exponents of its force. For example, if the pump, D, feeds the air into F, at 60 lbs. (approx. 4.1 bars) and the quantity contained in the pump is fed into the heater, and takes up 491° F (approx. 255° C), and then passes into the main cylinder: this is simply 16 cubic feet (approx. 453 l) of air at atmospheric pressure reduced to 4 cubic feet (approx. 133 l). Thus 16x15 (491° F) ÷ 4 = 60; and 15 x 4 = 60.
The question of compressed and non-compressed air, is just as broad as it is long, for it requires the same amount of power to compress it as is obtained afterwards from the same air in its compressed state, so that the simple question in relation to the power of any hot-air engine is resolved by the quantity of air at atmospheric pressure, heated to a certain temperature in a given time — the degree of heat determines the pressure, and the space through which it will move the piston.
When properly understood, the question is very simple. We regret to state that scientific men — Professors in some of our colleges — who have written on this subject, have involved it in mystery, by rushing into page after page of symbols and figures, to explain a question that requires only a very few figures in the most common rules of arithmetic. Calculating the effective force of hot air in a cylinder (under a certain pressure) at different points of the stroke — is labor lost in discussion, for such calculations merely relate to that economy of its use, which is equal to that of steam, and which is practiced in steam engines.
The great question to be asked in discussing hot air versus steam, is what advantage has air over steam? What is there in its nature that would render it superior as a motive agent to steam? It is far inferior to water raised into steam, as a motive agent.
The only single quality that it has, reasonably, over steam, is its inferior capacity for heat. Thus while the capacity of water for heat is 1.0000; air is only 0.2669, or 0.7331 less. But one cubic inch (approx. 17 cm3) of air heated to 210° F(approx. 99° C) will raise only 6.12 lbs. one inch, while 1 cubic inch (approx. 17 cm3) of water raised to steam at 212° F (100° C) will lift 15 lbs. 1728 inches.
Now let us suppose that the air is 815 times lighter than the water, and of 3.75 inferior capacity for heat, the advantage is still with the steam : thus 1728 x 15 = 255920 ÷ 815 = 31 ÷ 3.75 = 8, or about two pounds on the square inch (approx. 0.55 bar).
The great bulk of air, in comparison with that of water — it being 815 times lighter than water, is an objection to its use. it requires huge cylinders amounting to about 217 times greater frictional surface than steam engines. It acts chemically upon iron and oxydizes the parts exposed with great rapidity. The moisture of steam relieves the piston of much friction, and this is the reason why anhydrous steam (stame) when mixed with moist steam, produces better results than the stame.
Steam at the low temperature of 283° F (approx. 140° C) exerts a pressure of 50 lbs. on the square inch (approx. 3.4 bars), while air at 491° F (approx. 255° C) exerts one of only 15 lbs (approx. 1 bar). The steam boiler is a reservoir of force, not subject to those sudden changes involved in an air heater, when such an immense bulk of air has to be heated for every stroke of the piston.
Mr. Shaw is a sincere and honest explorer in this field. He presents his engine to the American public, and has courted a candid criticism, and for this he deserves the thanks of the community.