The Fumific Impeller
Source: Description of the Fumific Impeller; shewing the direct application of hot products of combustion to the bodies on which they are required to act, without intervention of machinery
Author: Alexander Gordon, Member of the Institution of Civil Engineers
Date: September 1845, 22 Fludyer Street, Whitehall,
Patent: dated March 3rd, specifications enrolled September 1845
The source of steam power is heat. A limited quantity is generated by the combustion of a limited quantity of fuel; and if this can be embodied and suddenly abstracted, it can be made applicable to locomotion, or any other purpose of mechanical agency.
It may, however, be embodied, and produce a powerful effect, although it cannot be suddenly abstracted, but then, of course, it is not applicable to machinery.
A beautiful instance of this motive power of heat occurs in the preservation of the Conservatoire des Arts et Métiers at Paris, the walls of which had begun, owing to the weight of the roof, to recede from each other. Iron bars, with screws and dises on each end, were inserted and passed through both walls; the bars were heated, and in their expanded state the dises were screwed up close to each wall.
In cooling, an enormous power was exerted by the contraction of the bars, which drew the walls back into their proper position. It is impossible to deny that heat is the cause of motive power in the steam-engine; and that power is not in proportion to the quantity of heat generated, but to the quantity which can be embodied in a state disposable for use.
That state in which it is yet known to us as most disposable for use is when the heat has been imparted to water, and formed the vapour of steam. Now water of one bulk, say a cubic inch of water, when formed into steam, occupies 1700 cubic inches; and it is by throwing heat into the single bulk of water, thus increasing its bulk 1700 times, and then by abstracting that heat with a cold application, so as to reduce the 1700 bulks to one bulk again, that power is produced in the low-pressure engine.
It is this generation and destruction of heat which produce the movement of dilatation and contraction. A chemical movement thus obtained is easily applied in a machine by the engineer, so as to maintain the power which can “engrave a seal, and crush a mass of obdurate metal like wax before it; draw out without breaking a thread as fine as gossamer, and lift a ship-of-war like a bauble in the air; which can embroider muslin, forge anchors, cut steel into ribands, and impel itself against the opposition of the very tempest.
Another mode of obtaining power in a steam-engine from heat imparted to water is by repulsion alone, that is to say, by high-pressures; and it is this modification of the steam-engine which is used for steam-carriages.
In the terms above cited, I expressed myself nearly fourteen years ago, and I therefore feel that I cannot better introduce the subject now to be explained, than by this reference to the first germ of it in my mind. It will be seen by the quotation that it is for no hastily conceived theory that I contend, and as my readers proceed they will admit the quotation to be a good point de depart for their future inquiry.
We have heard of the “golden age" and of the “iron age; the present may emphatically be designated the “age of steam.”
The construction and operation of the steam-engine, embracing, as it does, the most elegant and ingenious inventions and contrivances, are so interwoven with the interests of engineers and owners of steam-power, as they are also associated in the minds of all with the deservedly admired name of WATT, that the proposer in this day of a new inanimate agent of motion, must anticipate numerous difficulties.
In the steam-engine, water exists in every stage of its history or operation. The heat from the fire separates the ultimate molecules of the water, overcoming their mutual attraction, and endowing each of them with a force of repulsion in proportion to the amount of heat. Heat, then, is the source of that power.
All solids, liquids, and aeriform bodies, may be heated and cooled, expanded and contracted; very few of them, however, can be applied as an elemental power, and still fewer of them can be employed as a power for locomotion or navigation.
Some of the solids are used where power without speed is required, as in the case of iron bolts, rivets, ties, wedges, and in the iron tie-bars of the Conservatoire des Arts et Métiers just quoted. Some liquids, as for instance, spirits of wine, ether, ammoniacal liquor, liquid carbonic acid, have been proposed and experimented upon, with the view of being employed as means for an available motive power, by the alternate application and abstraction of heat; but water has hitherto been found the most convenient and economical means, for obtaining power from the chemical action of heat; and to this is due the general adoption of the steam-engine.
Many attempts have been made to employ air as the means for obtaining power from the chemical action of heat. Some inventors have followed the manner in which water is treated in the steam engine, by keeping the air altogether distinct from the fire, and transmitting the heat of the furnace through the materials of a tight chamber (like a boiler).
Of these the most successful have been the productions of Mr. Ericcson and Mr. Stirling. A full description of this elegant engine, with illustrative drawings, was presented to the Institution of Civil Engineers by Mr. Stirling, of Dundee (see here, Stirling Dunedee engine of 1842), and a long discussion, on its construction, action, and economy, took place, to which I beg to refer.
It will be seen that great difficulty was experienced by members in understanding the action of Mr. Stirling's air-engine. It appears to have been difficult of comprehension principally because the steam-engine is naturally taken by engineers as the standard of perfection.
Others have used the products of combustion by bringing them (without the intervention of any heating chambers like a boiler) at once to act in the piston-cylinder. This latter process was introduced in Sweden (Mr. Ericsson), and more recently has been carried into experimental operation by Sir George Cayley under his patent.
But, prior to either the Swedish engine or that of Sir George Cayley, Mr. Robert Stein, of Edinburgh, had, in August 1821, obtained a patent for improvements in steam-engines, and his improvements consisted principally in directing the actual products of combustion in combination with the steam, at once into the piston cylinder. Sometimes Mr. Stein dispensed with a boiler and water, and used only the products of combustion from the close furnace.
All of these, and all other inventors of hot air-engines, have taken the steam-engine piston and cylinder as their models; and, although several of them proved clearly that air is more economically heated to the required temperature than water, and, although Sir George Cayley proved this economy of fuel in an extensive practice, and Mr. Stirling has shewn the economy to be immense, no one has yet contrived how to maintain the durability, and, by consequence, the current economy of a hot air or caloric engine, when a piston and cylinder are operated upon at once by the products of combustion.
And of Mr. Stirling's engine it may be said, it bids fair to rival the steam-engine for manufacturing purposes, such as the impulsion of mill-machinery.
It is not necessary to enter into a tedious narrative of the hot air or caloric engines just referred to, nor to enumerate the various attempts to make hot aeriform bodies actuate a piston in a cylinder.
It may be asserted that hot aeriform bodies have not been successfully employed hitherto as the agents, because the great sensible heat has speedily destroyed some part of the machine; and because water has been preferred as the material, on account of the ease with which it can be procured, replaced, and managed.
It has been said before, and cannot be too often repeated here, that heat is the source of power. And it would be absurd to suppose that any intelligent person considered that the machinery, or any part of it, from the paddle-floats of a steam-boat, back to the boiler, really auguments the power which proceeds through the boiler and along the steam-pipe. The power, i.e. the combined mechanical agency of elasticity and momentum of which the steam consists, is within the steam-pipe. The engine, machinery, and paddles only offer the means of bringing that power to operate upon the water and urge the ship in her course.
The proposers of hot air engines have taken the steam-engine, subsequent to the discoveries of Newcomen, as their model, whilst they should have reverted at once to the engines of Savery and of Papin.
They should be referred to the Marquis of Worcester's scantlings, and even to the smokejack of Hieronymus Cardan.
The Marquis of Worcester' employed the pressure of steam to act at once and directly upon the water which he desired to put into motion. Savery, also, used steam in direct connexion with the water. Dennis Papin improved on these by interposing a loose floating piston between the steam and the water to be moved.
Now, had any one of these latter used, instead of steam, the hot products of combustion from a close furnace, the steam-engine would not now be the only available, inanimate artificial power in use for such purposes as raising water, and for locomotion and navigation.
I am well aware of the difficulties I must encounter; even the best steam-engine makers of the day being content with steam as the medium for availing of the motive power of heat. Born during the triumphs of that grand and beneficent agent of civilisation - educated in a mechanical school where it was the grand problem for their solution, - they grew with its growth, and have flourished under its power,- they may search out some improvements in its construction whereby fuel may be saved, and space and weight of the machine reduced; but they are so engrossed by the machine which they and their fathers have made, they have so lauded the powers, and so many of them have identified themselves with her multiform character, that she has verily become the “Goddess of their idolatry.”
There is therefore the more reason why I should address the general body of scientific readers. My invention and its value are embodied in three simple axiomatic propositions, which interested or ignorant opposition may challenge, but I defy it to overthrow.
-
That heat is the source of power in all our artificial motive agents.
-
That machinery does not increase the power of any machine, but only trans forms or transfers the motion.
- That the expansion arising from a given amount of heat applied to gaseous bodies is much greater than from the same amount of heat when applied to liquid bodies
The two first propositions will be admitted as correct by any tyro. We need not stop to examine them. Our discussion of the third proposition shall be as nearly as possible in the words of the best chemical authorities.
“It may be laid down as a general rule, to which there is no known exception, that every addition or abstraction of caloric makes a corresponding change in the bulk of the body, which has been subjected to the alteration in the quantity of its heat.”
From the experiments of Dalton, Gay Lussac, and others, it is well established that all gaseous bodies whatever, similarly circumstanced, undergo the same expansion by the same increments of heat; and Dr. Ure adds his testimony that “Expansions of aeriform bodies are proportionate to the augmentation of temperature;” and in the words of Dr. Thomson, the expansion and contraction differ exceedingly in different bodies. “In general, the expansion of gaseous bodies is greatest of all.”
Reference to any chemical authority for the proportional quantities of heat or fuel, which uniform weights of water and of air require, in order to have their temperatures raised the same number of degrees; that is to say, a reference to the undisputed specific heats of the two bodies, will prove this to be so.
The specific heat of water being 1, that of an equal weight of air is 0.2669, t therefore, if 1 lb. of fuel is required to heat a given weight of water 1°, the smaller quantity, 0.2669 of the same fuel will suffice to heat the same weight of air 1°.
Such is the result of the experiments on the specific heats of bodies by Lavoisier and Laplace, who burnt various bodies in the calorimeter, and estimated the heat by the quantity of ice melted in each experiment. These philosophers were followed by Crawford, Dalton, and Count Rumford; and the expense of heating water,is determined by them to be nearly four times the expense of heating air in careful laboratory experiments.
The value of one fuel or another for the purpose of generating heat is not a question here; but whether the body to be expanded by that heat should be water or air, is the matter before us.
Dr. Ure, in his “Dictionary of Chemistry,” art. Combustion, informs us that 1 lb. of charcoal melts on the average 68lbs. of ice; and by Turner's Chemistry (p. 57), 68lbs. of ice melted is equivalent to 68 × 140° = 9520 lbs. of water raised 1°. Hence it follows, that 1 lb. of this fuel raises 9520 lbs. of water 1°, or 74%, lbs. of water 1212°, or 19 lbs. of water 480°; and 1 lb. of same fuel raises 35575 lbs. of air 1°, or 29 lbs. of air 1212°, or 74 lbs. of
air, 480°.
Experiments made by the calorimeter lead us to believe that:
- 1 lb. of charcoal raises 9.520 lb. of water 1°
- 1 lb. of wood raises 6.300 lb. of water 1°
- 1 lb. of coke raises 13.068 lb. of water 1°
- 1 lb. of coal raises 6.534 lb. of water 1°
but in experiments by the calorimeter there is the mechanical obstacle of the body through which the heat has to be transmitted. In the fumific impeller I have no transmission; I have chemical union. The distinction is important.
There can, therefore, be no doubt that air, by Nature, is a better body than water to work with. But hitherto Art has not accomplished the manner of doing so.
Thoroughly impressed with the truth of the three propositions stated above, I made a course of experiments upon the action of the hot products of combustion when brought by rapid delivery into immediate contact with water. The success was so complete that I obtained a patent for an Improvement or Improvements in producing Motive Power by the action or agency of Heat, and in the application of that power to purposes of locomotion or navigation.
It was sealed 3d March, and the specification was lodged 3d September, 1845; and the following description is in strict accordance with the latter document, though stripped considerably of the legal phraseology in which specifications are necessarily worded.
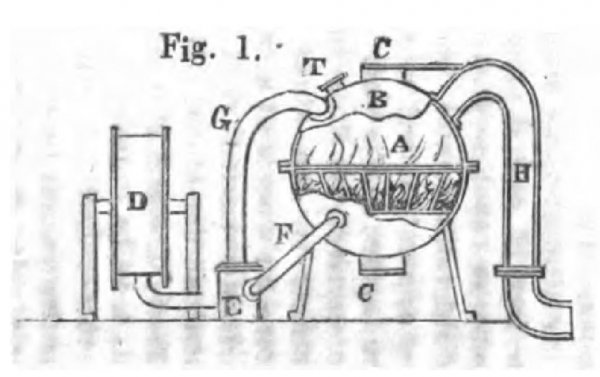
Fig. 1 is a sectional elevation of such an apparatus as will explain the nature and action of the Fumific Impeller. A is a fire grate inclosed in a strong close furnace B.B. There is on the top of this close chamber B a bonnet-valve C; and at the bottom there is another bonnet-valve C'.
B, the inside of this close furnace, is constructed of fire-clay, or fire-tile, and the external shell is formed of wrought-iron, of sufficient strength and sufficiently tight to resist the pressure from within. It might be made altogether of iron; but if the iron were not protected by some inner casing, it would not withstand for any great length of time the high temperature to which it must necessarily be exposed.
CC', these valves are kept tight upon their seats by means of the usual springs or weights, and not only serve for closing their respective apertures, but they are arranged so that they can blow open as safety-valves, when the pressure from within shall exceed the power of the springs or weights employed to keep them close.
T, is a pipe fitted in the close furnace, which, being provided with a talc-sight, enables the attendant to see the state of the fire.
The valves being open for the purpose of laying and lighting the fire, which may be of coal, wood, coke, charcoal, peat, or of any other fuel; and the fire having attained a good state of combustion, the valves C and C' are well luted and closed tightly on their respective openings.
D is a blower, which supplies atmospheric air to support the combustion in the close furnace. The air being directed by means of the valve E, so that it shall pass through the pipe For the pipe G; the former conducting it under or up through the fire, and the latter to the top and over the fire; thus the exact quantity to pass through or close to the fire may be regulated for consuming a quantity of fuel sufficient for producing the hot products required for the fumific influence; and thus the speed and power of the Fumific Impeller may be increased or diminished.
H is a pipe leading off from the top of the close furnace, by which the hot aeriform matters generated by the process of combustion are carried off to be applied in the manner which shall be explained more fully below; and these hot aeriform products of combustion are intermingled usually with some dust, ashes, and other solid matters, but which do not interrupt the dynamic action of the gaseous body.
Air, it is well known, will, when heated by one degree of Fahrenheit, expand about 1/480 part, and continue to expand so as to have its expansive force or tendency increased in about the same proportion for every additional degree of temperature.”
Dalton determined that 100 parts of air being heated from 55° to 212°, expanded to 132 5/10 parts; this gives us an expansion of 1/483 parts for 1° Fahr. Gay Lussac determined the expansion to be 1/480; and although in Sir David Brewster's edition of Robinson's philosophy, 24/10000, or about 1/417, is stated, we find Dr. Ure, in his “Dictionary of Arts” (article,“ Expansion”), states that all gases expand 1/480 for each degree of Fahrenheit.
It follows that if the temperature of a permanently elastic aeriform body be augmented by about 480°, the bulk of that body will be doubled, or if it be retained within the space it originally occupied, its pressure will be doubled. It is by availing myself of this well known law of expansion by heat and the new arrangement of particles in the close furnace, that I can obtain a rush of power from the furnace along the pipe H, analogous to the rush of steam from a steam-engine boiler along the steam-pipe to the engine, equal to it in pressure, power, and constancy, and, when required, at much greater velocity.
The air driven into B for support of combustion must, of course, be driven in against the pressure due to the heat of the products of combustion ; for, supposing the latter are at the temperature of about 500°, there will be an atmosphere of surplus pressure against the blower; and it will be found that the blower, to do its work, will require a power of 3 the power generated by the heat.
The manner in which this blower is worked, and also the manner of starting the engine, are shewn below. It has been shewn previously, that the power of a steam-engine is from the fire “through the boiler and along the steam-pipe; that the power, - i.e. the combined mechanical agency of elasticity and momentum, of which the steam consists - is within the steam-pipe.”
Now, in my Fumific Impeller, the power, - i.e. the combined mechanical agency of elasticity and momentum - is in the hot products of combustion themselves, which are in the pipe H.
In a steam-engine, the power of heat is necessarily transmitted through a costly and elegant system of machinery to act upon the bodies, which it moves or which it moves upon. The steam being carefully prevented from contact with any body but its own engine.
In the Fumific Impeller, I bring the power of heat, without the aid of any transmitting machinery, to act directly and at once upon the body or bodies to be put in motion, or on the medium or media in, or on, or through which the ship, or machine, or carriage, is to be moved or impelled; and having hot products of combustion as my power (not the easily condensed and tender steam), I allow them to come into immediate conduct with such bodies.
A stream or streams of the hot products of combustion are discharged under water backwards, and the vessel moves forward, or they are discharged forward, and the ship is moved backward. It is a case of recoil analogous to that which takes place in a rocket occasioning its flight, but differing from that of the deadly missile, in being manageable and safe, as we shall presently see.
When a gaseous current rushes uniformly from the enclosed space where it is generated, the dynamic power of the current is equal to the pressure exercised by the gaseous body at the orifice of escape, multiplied by the speed by which the body is generated and driven out.
According to this principle, let us suppose that the fumific influence imprisoned be two atmospheres = 2 a in the close furnace, and that it flows directly into the surrounding medium of water with a velocity = v.
If b represent the area of the orifice of discharge, the pressure exercised by the fumific influence will be represented by bav.
In any case, the fumific impulse delivered under water will require less diameter of discharge-pipe than will be required for the main steam-pipe of a steam-engine of the same nominal horse power.
The action of a rocket is not generally appreciated, and the value of the discharge of the hot products of combustion from the Congreve rocket, which is impelled by their action upon the surrounding atmosphere, is so little known that, at the risk of being tedious, I shall venture to say a few words on this prototype of dynamic impelling agency without machinery.
The whole subject of congreve rocket manufacture is kept most jealously secret in the government arsenals; still the flight of the missiles may be witnessed on great occasions at Plumstead Marshes, and I am enabled to state a little more than is generally known of their projectile power.
One of the largest now used in the English service, for either bombardment or field-work, is called a 32-pounder Congreve rocket. The entire weight, with composition, clay, iron, stick, and the gunpowder for bursting the cast-iron head at the end of the range, is 33 lbs. The length of the head and case is 2 feet, the external diameter 8, inches.
The rocket-composition occupies an interior cylindrical space of 33 inches diameter x 21 inches long; and this composition weighs 10 lbs.
The combustion of this 10 lbs. of composition and its rush a tergo projects the rocket over a base line of 3450 yards (or only 70 yards short of two miles) in seven seconds.
Here, then, is an instance of much higher velocity than that of any machinery or implement, not even excepting a cannon-ball. “The resisting body in the case of the rocket,” as in the case of a piece of ordnance fired with a wad, as is done in Dr. Hutton's pendulum, “is the air; and the flight of the rocket thus depends on a comparison between the weight to be moved (adding to it the effect of gravity and the anterior resistance of the atmosphere) and that of the resistance which the air opposes to the issuing current.”
“We have here assumed grounds of computation which are not exact, but quite sufficient to explain the general principle."
“The composition produces 500 times its bulk of gas at the mean temperature of the air, whilst its elastic force is increased by the heat to not less than 2000.”
“In water we overcome the inertia of the body to be moved more quickly than in the case of a rocket in the air, the water against which the discharge takes effect is of so much greater specific gravity than the air.”
When a rocket pitches into a sandbank or a rampart before the impelling composition has been exhausted, the sand falls in behind it, and offers || resistance to the discharge, so that the rocket continues to burrow under ground as long as there is any composition left to generate fumific influence.
The impelling composition of a rocket consists of combustibles and a supporter of combustion, selected, proportioned, and intimately mixed for augmenting volume by a fresh arrangement of particles, and for producing the aeriform fluid at a high temperature.
There is a mass of this intimate mixture within the case, which, independent of any oxygen from the atmosphere, forms in a few seconds into a fumific impulse, which discharges itself through what is called the “throat” of the rocket.
In my Fumific Impeller, I only allow the coals and the atmospheric air (or the combustibles and the supporter) to meet when and as the chemical energy is required; the object is not to shoot a boat off at the rate of two miles in seven seconds of time, but at known and ordinary velocities; it is not to send it bodily into air, where, as in the case of the rocket, it would have to struggle against gravitation. The boat is borne by the water.
The paddle-floats of a steam-ship overcome the inertia of the mass by a perceptible succession of blows on the water. The
Archimedean screw accomplishes the same, and by a less perceptible succession of impacts. The flow of fumific power will accomplish it by an imperceptible succession of impacts from innumerable molecules.
Let us now see how a ship may be fitted and actuated by the Fumific Impeller.
Fig. 2 is a longitudinal view of the midships and after-part of the ship with the midship part shewn in section.

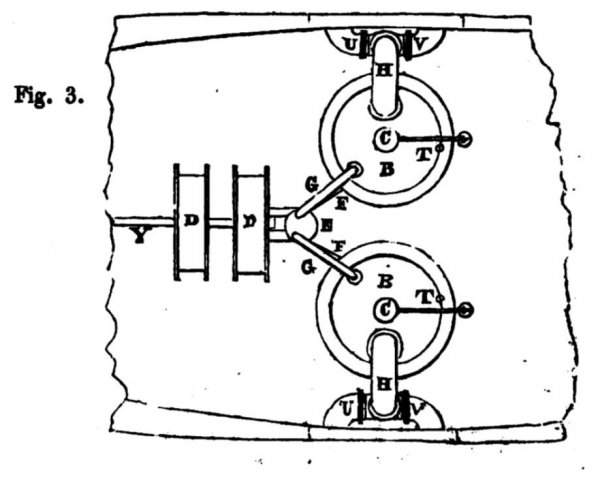
Fig. 3 is a plan of fig. 2,
and fig. 4 is a cross section of the same.
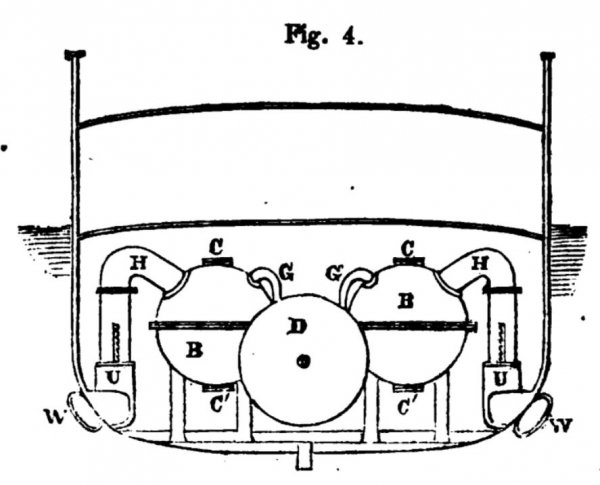
The functions, actions, and mutual relations of the fires: the close furnaces, B B ; the valves, C C C C'; the blowers, D D; the cocks or valves, E E ; the pipes, FF and G G ; and the pipes H H, which lead off the power from the close furnaces, need not be described here. They will be understood to be a double arrangement of what has just been shewn and explained in fig. 1.
The products of combustion intermingled with any dust, ashes, or other solid matters (even cinders and clinkers), are caused to rush along the pipe H on each side of the ship, and through one or other of the valves U or V directly into the water. If, on the larboard side, the valve U be opened, and the valve V be shut, while the valve U on the starboard side is open and the valve V shut, the products of combustion must then rush out at the discharge pipes WW, and overcome the inertia of the ship, and the continuous flow of power from W continues the onward movement of the ship.
The ship having been put into motion, a fan or screw X in the dead wood of the ship is, by the motion of the ship, caused to revolve, and its shaft Y gives motion to the tight blowers or air pumps at D D.
There are many other arrangements of paddles or screws which may be adapted for any of the numerous parts of the ship, and may be shipped when required for appropriating a part of the motion given to the ship, and unshipped when not required for such purpose.
As the valves UU may be used for impelling the ship forward, so the valves V V may be employed to back the ship; and the valve U on one side of the ship, and the valve V on the other side of the ship, may be employed to put the ship about.
The pipes branching from the valves U and V may be kept inside of the ship, and made to terminate and discharge the power when the moulding of the bottom bilges or other submerged part of the ship may intersect them. ZZ is a folding chimney with a sliding part at the lower end for carrying away the smoke and vapour when the valves C and C are opened.
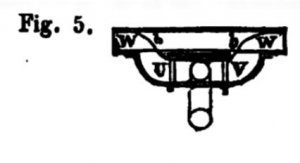
Fig. 5 shows a plan of the interior of the discharge pipes leading from the valves U and V. The curved partitions t t are useful to direct the current forward or aft as may be required.
To put the impeller in motion a few cubic inches of water are injected, by a small pump or hopper, into the fire, which water flashing into an aeriform state (partially or wholly decomposed) gives the first stroke or strokes to the Impeller. Or, instead of water, a solution of nitre, which will also be found very useful in getting the fire into greater activity.
The combustion of the fuel may be accelerated, and also additional heat be obtained, by injecting into the closed furnace from time to time, by means of the blower D or any other blower, some coal-dust, or powdered rosin, or other pulverised combustible or supporter of combustion. But whether such materials are blown in singly or together, they should be introduced into a cold part of the blast-pipe, blown or dropped upon the fire, not passed up through it. Of these auxiliary materials coal-dust will, perhaps, be found the best to employ, for it can be readily obtained; and, as it inflames rapidly, there is, consequently, a rapid augmentation of the volume of heated air and vapours.
Sometimes these auxiliary materials may be forced into the close furnace by means of a hopper, which may be kept air-tight by a double mouth-piece, or the hopper may deliver the said auxiliary materials by means of a cock half perforated or a fluted roller.
One or more short tubes T fixed in the upper part of each furnace (with eyepieces of talc for the purpose of keeping in the pressure) serve to sight the condition of the fire.
The bonnets or valves C and C’ can be opened as often as necessary in order to clear the fire-grate or to supply fresh fuel, and they are rendered tight by means of asbestos and white or red lead as a luting, or by means of other good luting. Sometimes a close hopper or hoppers with double mouth-piece may be adopted for supplying the whole of the fuel to the fire, and sometimes it is advantageous to supply through such hopper a mixture of coals and water.
The proportions, between the area of the fire-grate or fire space and the area of the pipe H, to be preferred, are about the proportions at present adopted by makers of marine steam-engines in their fire-grates and main steam-pipes. But the Fumific Impeller may be worked with a smaller proportion of fire-grate; because, not being delayed by the process of transmission of heat through metal (as is the case in a steam boiler), but being as rapid in the generation of power, as is the appropriation by fire of oxygen from the air blown in, there can be no other limit set to its speed.
It will now be easily seen how a locomotive engine may be impelled by this motive agent.
Figs. 6, 7, and 8, exhibit sketches of an application of my Fumific Impeller to locomotive or railroad purposes;
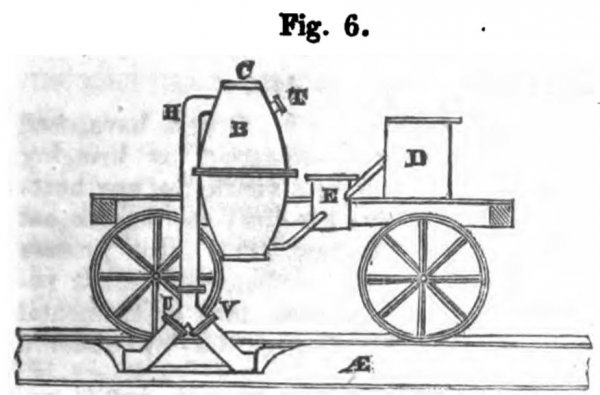
fig. 7 being a longitudinal section;
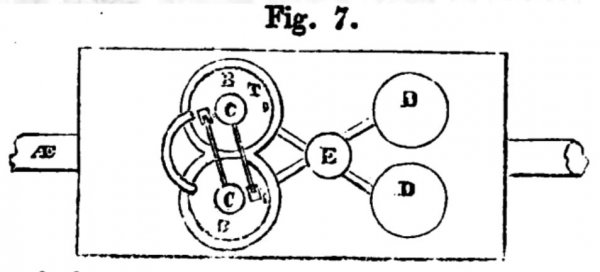
fig. 8 a plan, and
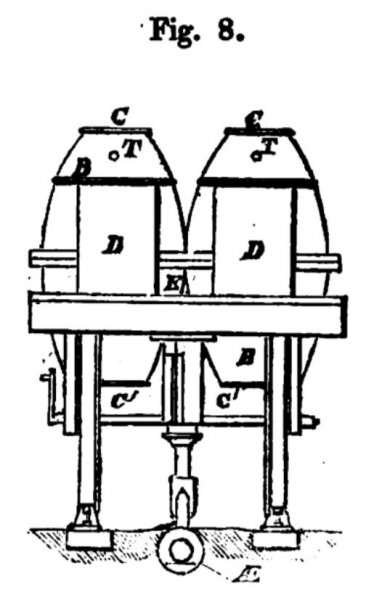
fig. 9 a cross section. The general arrangement of the close furnaces, air-pumps, and pipes, common to my Fumific Impeller, need not be described again here.

The four or more wheels of the locomotive are placed upon the rails. AE is a continuous tube or pipe similar to the pipes for atmospheric railway communication. Through the continuous opening, at the upper part of the tube AE, the pipe H is made to discharge the hot products of combustion, either through the valve U to impel the locomotive forward, or through the valve V to impel the locomotive backward, the discharge pipe from U delivering the hot products of combustion, the impact is got upon the air or aeriform fluid in the pipe AE.
The use of this pipe AE being principally to prevent the lateral escape of the impelling and the resisting bodies, it may be made of iron or other suitable metal, or it may be constructed in masonry.
Instead of the continuous tube, as shewn on figs. 7 and 8, a continuous trough of water or canal may be under or at the side of the railway, and the pipe H and delivering apparatus may be led so as to act under the surface of the water, whilst the weight to be carried, the close furnace and other machinery or apparatus are transported or borne on wheels or a carriage.
It is obvious that by causing an arrangement similar to the above described (in figs. 6, 7, and 8) to be employed in a carriage, to be impelled on a road surface other than a railway, or upon turf, or other way, the inertia may be overcome and the carriage maintained in motion by the projection of the products of combustion backwards when the carriage is to be impelled forward, and forward when the carriage is to be backed. But in this case I prefer not to depend upon the products of combustion driven wholly into the surrounding atmosphere, but to force them diagonally downwards, so that impact may be had upon the road or way.
In case of locomotion the air-pump is actuated by the revolution of the axle.
Fig. 9 is a sketch of an apparatus wherein the Fumific Impeller is applied to the raising of water from one level to another. J is a cylindrical working chamber which has been nearly filled with water from the level K through the pipe k, and communicates at top with the pipe H. When the hot products of combustion enter J, their pressure on the surface of the contained water, closes the inlet valve L of the pipe k, and forces the water up the pipe M to the higher level N.
We have seen previously, that part of the power obtained by the hot products of combustion must be used for pumping air into the closed furnace. When water is pumped up, part of it must be used to work the pump ; therefore, from N a portion of the water is returned back by the channel o to actuate a water wheel O which works the blower D. The same water-wheel works the valves Q and S, which are necessary when the working chamber J is used. When the water in the working chamber has been depressed by the pressure to the level J', the water-wheel O opens the valve Q of a waste pipe R to permit the escape of the now expanded aeriform products, and closes simultaneously a valve h which commands the mouth of the pipe H. The working chamber J is now refilled with water from the level K by the superior pressure of the, water in the direction of the pipe k and the valve L, and as soon as refilled (or nearly so), the water-wheel again opens the valve h, and the process of forcing up the water to N, above described, is repeated.
On the top of the water in J there may be a float in one or more pieces of stone, wood, or hollow metal P, to prevent or lessen the splashing of water, and at the bottom of the chamber J there is a trap and man-hole door b, through which the ashes, cinders, and other solid matters which collect there may from time to time be withdrawn.
When only one close furnace B and one working chamber J are employed, it is necessary that the blast of atmospheric air should be also regulated by the water-wheel O, or by manual, or other power. But it is preferable to work with two close furnaces, two working chambers, and two blowers (as shown in plan, fig. 10), whereby a continuous action will be obtained.
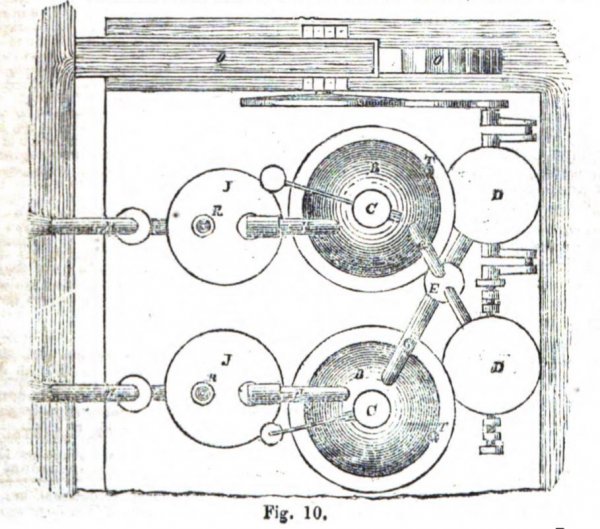
And in this latter case one water-wheel is sufficient for blowing the air and working the valves.
To start this engine it will suffice to use some water from the higher level N to set the water-wheel and blower in motion, but should there be no water in the higher level N, it will be necessary to inject a small quantity of water or solution of nitre into the close furnace, as explained in describing fig. 1. A branch pipe from the rising main M may be laid so as to throw some of the first water raised by J upon the water-wheel.
Readers acquainted with the early history of the steam-engine will recognise in the working chamber J of this engine (figs. 9 and10), an action of hot air similar to the action of steam in the proposition of the Marquis of Worcester, and in the practice of Savery and of Papin, as they are described by Tredgold and by Farey.
Having now described the construction and action of the Fumific Impeller for purposes of navigation, locomotion, and the raising of water, it may be well to anticipate the objection that half the fumific influence obtained will be required to maintain the blast. Any one making such an objection must have lost sight, for the time, of the specific heat of air being 0.2669, and the specific heat of water being 1.0 (stated above), and of the fact that consequently a certain amount of fuel is necessary to expand the molecules of water, giving it a temperature of 1000° latent heat and 220° sensible heat = 1220°, at a cost, say, of 20 shillings; whilst only 269/1000 of the same heat will raise air to the same temperature of 1220°, i.e. at a cost of 5 shillings and 38/100 of a shilling; add the half of this price for the air-blast, viz. 2 and 69/100, and the cost will be only 8 shillings and 7/100 of a shilling.
So that if a steam engine could be made to use, and use profitably, all the heat generated in its furnace, that heat, or power, would cost 20 shillings, whilst the same heat, or power, in the Fumific Impeller would only cost about 8 shillings: a sufficiently decisive proof of the economy of the power sought to be applied.
It must be borne in mind that, whilst by using the entire products of combustion and only losing a little by radiation, the Fumific Impeller has an immense advantage over the steam-engine, which cannot employ the whole of the heat generated. The hot smoke and aeriform products escape at the top of the chimney at a high temperature. The waste water carries off some more of the heat in addition to losses by radiation, etc., and there is a very large demand for power (heat) to drive the constituent parts of the engine.*
From the quantity of uncondensed steam, the friction of the parts, and other sources of resistance in steam-engines, the power is generally reduced about one-half in its effective operation as a moving power (Practical Mechanic's Pocket Guide, Glasgow, at page 45).
Mr. Andrew Murray, assistant engineer of Woolwich Dockyard, has investigated the combustion in marine steam-engines, and considers that the mean temperature of the furnace of a steam-boat boiler is 1000°, and that at the top of their chimneys 500° of heat are lost, and we have seen above that 500° constitute an atmosphere of pressure.
Mr. Murray has ably investigated the quantity of air chemically required for the perfect combustion of a given quantity of coal, of the quality commonly used for steam purposes; and states the amount of air chemically required for the combustion of one pound of coal to be 150.35 cubic feet, of which 44.64 cubic feet are required for the various carburetted hydrogen gases given off, and 105.71 for the solid carbon; he shows, however, that
“the practical utility of this knowledge is much impaired by the circumstance, that combustion ceases even in pure oxygen, and much more in air, before the whole of the oxygen present has entered into the new chemical combinations required. It is also known that carbonic acid gas exerts a positive influence in checking combustion, as a candle will not burn in a mixture composed of four measures of air and one measure of carbonic acid gas.
Large quantities of gas being generated by the combustion of the solid carbon on the grate, and being necessarily mechanically mixed with the inflammable gases as they rise, the quantity of air required for their subsequent combustion must, on this account, be increased to a very large extent.
The whole of the air thus supplied in excess must be heated to a very high temperature before any combustion can take place; and the loss of the heat thus absorbed must be taken into account in calculating the ultimate economy of igniting these gases.”
It is not necessary to discuss here the comparative values of different kinds of fuel, nor to state how many more combustibles and supporters of combustion may be used with advantage in the Fumific Impeller, than can be used in the steam-engine.
M. Despretz finds that when equal quantities of oxygen are used for the combustion of the following substances, the annexed proportions of heat are developed:
- Hydrogen: 2578°
- Charcoal: 2967°
- Iron: 5325°
Phosphorus, zinc, and tin give nearly the same quantities as iron. It appears then, that hydrogen developes the least heat for the same proportion of oxygen gas absorbed, the metals disengage the most. It is remarkable that carbon, which does not alter the volume of gas, evolves a quantity of heat, which is equal to of that given out by iron and the metals in general. (Annales de Chimie, Feb. 1828)
In the steam-engine, the heat being transmitted to the water in the boiler, it is found more economical to have large fire and slow combustion. In the Fumific Impeller, the chemical truth comes out that the more rapid the combustion the greater the economy of heat.
The subject cannot be dismissed without a glance at the great saving of weight and the greater safety, which must result if aeriform products of combustion, with the necessary furnaces, pumps, and pipes, &c., be used instead of water and all the machinery of the steam-engine.
A marine steam-engine of 500 horse power, of the lightest construction, weighs not less than 250 tons, with water in the boilers, say 295 tons. Is it too much to expect that a Fumific Impeller may be had of the same power to weigh two-thirds less, and occupy much smaller space? Is it too much to expect a saving of the 500° (i.e. half the fuel) which we have seen (above) Mr. Murray assigns as the loss at the chimney-top?
May we not also expect the saving of part of the cost of the other moiety, a part of that saving due to the difference of the specific heat of air and of water (stated above)? I trust not. In any case there is a wide margin to work upon.
The passage above quoted (see introduction), from the “Treatise on Locomotion,” 1832, mentions that portion of the heat which can, in the case of the steam-engine, be easily embodied in a state disposable for use; but the Fumific Impeller uses the whole of the heat generated, the entire products of combustion.
One feature in the power which I am now desirous of promoting is the greater safety of using the hot products of combustion as a prime mover, instead of using water and its steam.
In a steam-engine boiler the heat imparted by the furnace is given to every molecule of water inclosed in that boiler, much as carbonic acid gas is forced mechanically into soda water. When the boiler is closed at its valves and openings, the water may be at 220° or 250° of sensible heat, or at a lower or at a higher temperature (suppose the fire removed from under the boiler, the heat is still in the boiler); the water which is possessed of the heat remains tranquil, the steam is colourless, invisible.
So also in a tight bottle of soda-water, the water is possessed of the carbonic acid gas; all is quiet, no ebullition, no effervescence. But start the cork, a report is heard, a rush from within takes place, and every particle of the soda-water gives off suddenly its carbonic acid from the bottom, the top, the sides, and from innumerable points. In a hot day, and with a warm hand, the bottle may be held upright, and yet empty itself almost entirely. If, instead of allowing the cork to spring out suddenly, we gently allow the carbonic acid to flow out, ebullition is apparent, but a report and loss of the soda-water is prevented.
Now, in the case of a steam-engine boiler, open the main steam-pipe or a safety-valve gently, and steam comes off; but let the steam-pipe be kept close, the engine not going, the safety valve accidentally or designedly kept close;— suppose a defective plate or seam in the boiler, the weaker part gives way, not gently but suddenly, and every particle of hot water flashes into steam.
A boiler of 100 horse power for marine purposes has, say 9 tons of water charged with heat; when it bursts, this water, which occupied as a liquid, a space in the boiler equal to only 3580 cubic feet, starts out into a bulk of more than 6,086,000 cubic feet. The terrible effects are not limited to the first impact which rends the boiler; there is a rapid succession of impacts, each particle of water giving out its power; and hence it is that sometimes half a boiler, weighing ten or fifteen tons, is projected to a great distance.
Artillerymen know the value of a succession of impacts. They never use pistol-powder to project a heavy round shot or bombshell. They use a large grained powder, which, by inflaming more slowly, gives time to put the heavy mass into motion, and follows it up the chase.
In the boiler there is an immense reservoir of power. In the close furnace of the Fumific Impeller, the contained bulk, the products of combustion at a temperature of 500°, is not only much smaller, but (it cannot flash into 1700 times its bulk as water does) it only, when liberated, occupies double its bulk; if the temperature in the Fumific Impeller were even as high as 1020°, it could, when liberated, only occupy about treble its bulk. The bursting of the close furnace would be a mere flash in the pan, that of a steam-boiler, the explosion of a magazine.
With these remarks, founded on facts well established, and laws which are unalterable, I submit the Fumific Impeller to the notice of the public. They may or may not agree that the manner in which I propose to avail of the fumific impulse is the best; at all events, they will see the subject to be one of deep interest.
The measure of the worth of the present, or of any other invention, or discovery, in natural or mechanical philosophy, is to be estimated by its general utility.
The best system of raising water, or of propelling ships or locomotive engines, is that which presents the least danger, the greatest economy, comfort, and commercial and political advantage, and the best means for working at any required velocity; which shall cost the least sum for its establishment, exercise, and maintenance.
On every one of the required conditions, I have the strongest conviction of the superiority of the system here explained for the more prominent purposes of drainage, navigation, and locomotion, and I entertain no doubt of its applicability in affording, with equal advantage, a steady prime-mover for mill and manufacturing work in many situations. Attentive, intelligent, and impartial readers will, I am confident, arrive at the same conviction.
My early adoption of the proposition, that heat is the source of power in elemental agency, has been already shewn; a consistent adherence to its truth has been maintained. The applications of that fundamental law have just been explained.
As an engineer, known to have no small practical acquaintance with steam-engines, machinery, and steam-ships, and with an extensive practical knowledge of pneumatics, I trust I shall avoid any accusation of presumption in asserting that in this pamphlet the advantages of bringing the hot products of combustion to act directly and at once on liquid or on aeriform bodies, are indicated truly at least; and a short time will see the views herein propounded practically brought into successful operation.
The public mind opens but slowly to the adoption of any topic so simple and so entirely new as that which I have explained ; yet this master movement, the dynamic action of heat upon solids, liquids, and aeriform bodies,—remains in daily operation, and may be traced in the occurrences of ordinary life. I confidently expect the present generation will see an enlarged application of that agent, so beneficently committed to man, and that it will roll the tide of truth, of human life, and of human industry, to the most difficult and the most distant sites of our extensive commerce or philanthropic embassage.