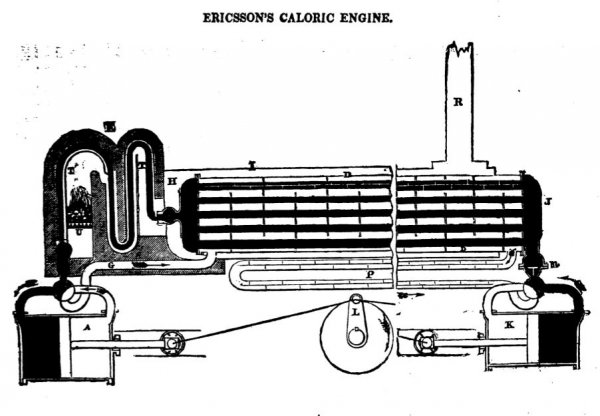
Ericsson's description
Source: Mechanic's Magazine, Museum, Register, Journals and Gazette, Vol XX
Date: Edward-street, Regent’ Park, January 7th, 1834
Title: Farther illustration of the principle of Mr. Ericsson's Caloric Engine
Author: John Ericsson
Sir, The following remarks, in elucidation of the principle of my Caloric Engine, will, I feel confident, not be unacceptable to your many scientific readers.
To arrive at a clear understanding of the advantage gained by the new mode of employing heat adopted in this engine, it may not be amiss to pause for a moment to consider how heat is at present made use of when employed to actuate that universal instrument of mechanical power, the steam engine. Is it necessary to the effect produced that the heat should be absorbed or destroyed, or in any way diminished in energy?
If this question can be answered in the negative, then it will be quite logical to assume that the power of the steam engine forms but a fraction of that which the combustion of a given quantity of fuel is capable of producing. Well, then, let us suppose a quantity of steam, of known volume and pressure, to be admitted into a vessel containing cold water of a given weight and temperature, the elevation of temperature which will be produced will, of course, afford an accurate measure of the quantity of heat contained in the steam previous to its condensation.
Suppose, now, that an equal volume of steam, of equal pressure, as in the first instance, is admitted under a piston, working in a cylinder, and subjected to a proportionate load, that piston will, of course, move until all the steam has been admitted, and, by its motion, exert a force propor tionate to the pressure of the steam and the volume displaced.
Let, then, the steam be discharged from under the piston into the vessel of cold water, under similar circumstances as in the first sup position, and it will be found that the same elevation of temperature will take place as when the steam was not previously employed to raise the piston.
We thus find that the production of mechanical force by heat is unaccompanied by any loss of heat (Losses by radiation need not here be taken into account, for they do not affect the theory).
But, in the steam engine, this remarkable circumstance is not productive of any advantage, for although nearly all the heat generated in the boiler is unquestionably conducted to the condenser, that heat cannot from thence be brought back to the boiler again for the purpose of raising steam, having in the condensing process been diffused amongst a large quantity of matter, and brought to a much lower temperature than the steam.
Of course, every boiler is fed from the condenser, but this produces a saying of fuel of only one-thirteenth part of the whole quantity consumed, hence thirteen fourteenths of the heat generated is constantiy wasted.
On these grounds the inference seems incontestible, that the steam engine is not constructed on a correct physical principle, inasmuch as it consumes a greater quantity of that precious commodity, fuel, than is necessary for the production of the mechanical force obtained.
It is well known that all fluid substances, the gases particularly, expand very considerably by being exposed to the action of heat, and that, if kept in a state of compression previous to being heated, their expansive force will, at a given temperature, be greater, and that in the same proportion as the increase of density.
That an engine might be worked by means of such expansion or dilatation, will be readily admitted by any one reflecting on the subject, without referring to the diagram or sketch of the Caloric Engine given in a recent number of your Magazine. I will, therefore, not detain your readers by detailing the manner in which the motion is practically produced by the dilatation of the heated medium, but confine myself to the theory of the contrivance, by which a nearly unlimited quantity of the impelling medium (gaseous or fluid) may be heated to any required temperature, by the consumption of a small quantity of fuel.
The journal cited in my last communication, having, by some strange oversight, mistaken the Caloric Engine for an “Air-Engine,” it will be well to direct the attention of your readers to the fact that various gaseous and even fluid substances capable of considerable dilatation by heat, are equally applicable for using the heat over and over again; and for the reason that the impelling agent may be varied, while, in every case, caloric is indispensable, has the term Caloric Engine been chosen.
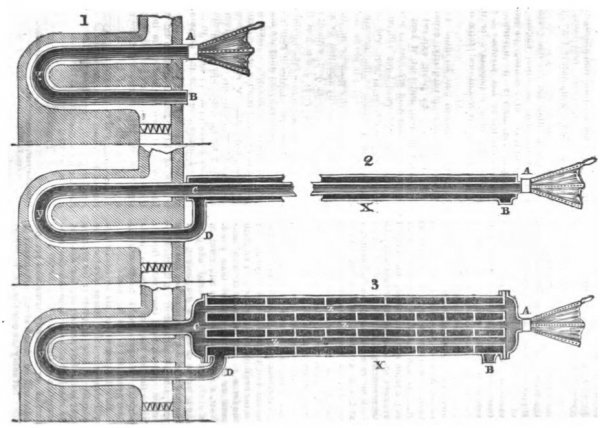
Let fig. 1 (see the accompanying engraving) represent a furnace having a metal tube y, conducted through the centre of its flue, to be acted on by the heat in its passage to the chimney; let a pair of bellows be attached to the pipe y at A, for the purpose of keeping up a constant current of air through that pipe; and let a thermometer be inserted into it at A, and another thermometer at B.
Now, suppose a regular fire to be kept up, and the bellows to be regularly worked so as to blow, say 20 cubic feet of cold air into the pipe y per minute: if it then be found that whilst the thermometer at A indicates 60° the thermometer at B will continue to indicate 100°, it follows, as a matter of course, that the heat transmitted by the furnace per minute will be accurately ascertained by calculating what quantity of heat is required to raise 20 cubic feet of air from 60° up to 100°.
Now, suppose the same furnace, with its metal tube, to be represented by fig. 2, but instead of having the bellows attached to the metal tube, suppose them to be attached to a pipe, AC of infinite length, and let this pipe be inclosed in a casing X; suppose farther this casing to be surrounded by a perfect non conductor of heat, and instead of allowing the hot air to pass off directly, as at B, in fig. 1, let it be conducted from the metal tube y, through the pipe D, into the casing X, and pass off at B. Then let thermometers be inserted in the pipes at A, C, D, and B, the bellows being worked at the same speed as before, and an equal fire kept up.
At the commencement, the thermometer, at A and at C will of course both indicate 60°, but the thermometer at C will very soon begin to rise, on account of the heat conveyed into the casing X; but any increase of temperature at C, will of course cause an increase of temperature at D. This again will still further increase the temperature at C, and so on, in continued succession, until the thermometer at D indicates a temperature nearly equal to that of the hot air in the beginning of the flue leading from the furnace: any further increase of temperature of course cannot take place.
Now, since the quantity, or rather weight of air forced through the metal tube y, is the same as in the first proposition, and the power of the fire likewise, this latter proposition, illustrated by fig. 2, incontrovertibly proves, that the temperature to which the air may be brought is made perfectly independent of the quantity of heat generated in the furnace.
But the quantity of air to be heated will also be equally independent of the quantity of heat generated: for suppose that in the first proposition, the draught be checked so as to diminish the consumption of fuel 3/4ths, then the 20 cubic feet of air constantly circulated per mi nute will be raised about 10° instead of 40°; but apply the contrivance for bringing the heat back, as illustrated in fig. 2, and the thermometers at C and at D will be affected just as above described, except that more time will be required before the temperature at D is brought to the full height, and that less heat will ultimately escape at B.
Thus it may be proved theoretically, that any quantity of fluid air or gaseous matter can be heated up to a high temperature, independently of the quantity of heat actually generated for that purpose. Although this is apparently a paradox, it is not so; for by referring to the illustration in figs. 2 and 3, it will at once be seen that the circulating fluid is of a high temperature only when passing the point D, and that it gradually diminishes in temperature, as it recedes and gradually increases as it advances towards that point. However, for the purpose of obtaining mechanical force, this is quite as advantageous as if the fluid retained its high temperature when it escapes; for at the point D is the heated fluid admitted into the working cylinder, and from thence passed off into the casing X.
The manner in which this is done, your diagram of the Caloric Engine, in a former number, fully explains.
Fig. 3 represents the form of apparatus used in practice; its operation is precisely the same as in fig. 2, and thermometers placed at A, C, D, and B, will indicate temperatures, proving the increase of temperature and transfer of heat in a similar manner.
The cold fluid is forced into the furnace through a number of small tubes Z, and the hot air is passed off through the vessel X (called the regenerator). The currents, both in this vessel and in the tubes, are broken in a peculiar manner, so as to produce a constant intermixture of particles, which is absolutely necessary for effecting a rapid transfer of heat. But to such an extent has this object been attained by the contrivances instituted, that hot air, constantly passed at the rate of 6 feet per second, through a pipe of 1 1/4 inch bore, 14 feet long, and entering at a temperature of 300°, has, by a counter-current of equal magnitude, been brought down to 85°, the counter-current at the same time entering at 72°.
I remain, Sir, yours, &c.
J. Ericsson